Description
Structural Interventions: Congenital Heart Defects
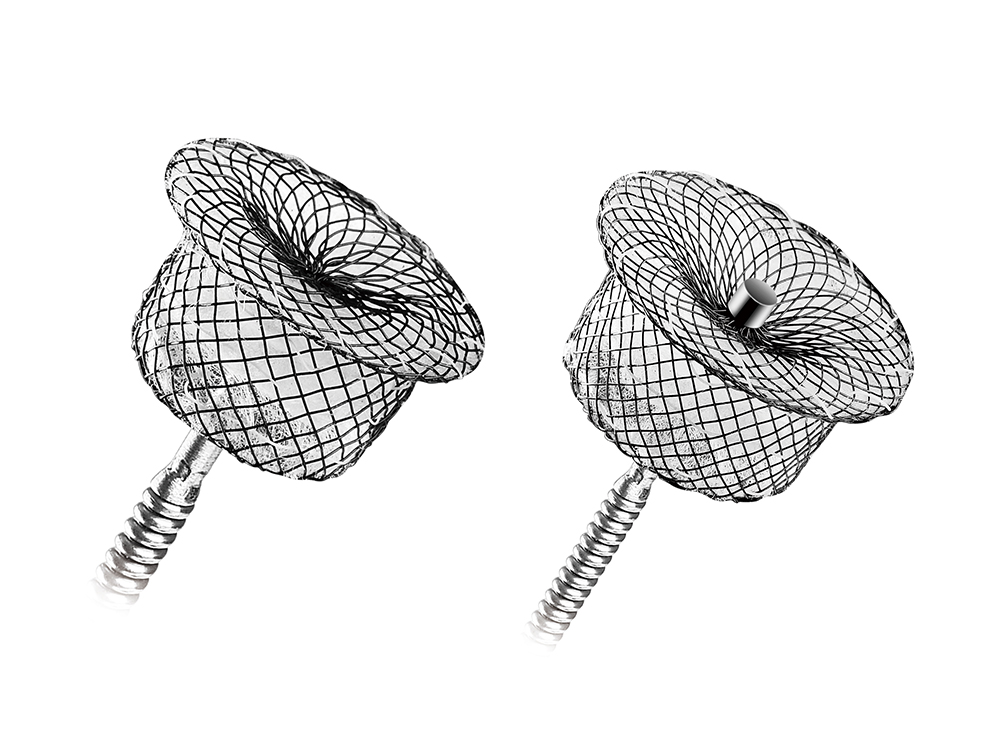
Amplatze Duct OccludersThe Amplatzer Family of Duct Occluders offers an extensive line of devices and a simple treatment option for the closure of patent ductus arteriosus (PDA). Amplatzer Duct Occluders are designed to occlude PDAs of various shapes and sizes. With an easy-to-perform deployment procedure, the implanting physician can be confident in placing the device with precision.
|
 |
Amplatzer Septal OccludersVentricular septal defects (VSD) are the most commonly found congenital heart defect. One subtype of these defects is muscular VSD. If left untreated it can lead to pulmonary hypertension and/or congestive heart failure. The Amplatzer Muscular VSD Occluders are designed for complete closure of these type of defects. |
 |
Amplatze Muscular VSD OccludersAmplatzer Septal Occluders are the standard of care for minimally invasive atrial septal defect (ASD) closure. These double-disc occluders are comprised of nitinol mesh and polyester material. They are designed to securely appose the septal wall on each side of the defect and create a platform for tissue in-growth after implantation. |
 |
Structural Interventions: Stroke Risk Reduction
Amplatzer Talisman PFO OccluderThe Amplatzer Talisman PFO Occluder set the standard, pioneering treatment with a device developed specifically for patent foramen ovale (PFO) closure to reduce the risk of recurrent ischemic stroke. It’s the leading PFO closure device to date, with over 180,000 devices implanted worldwide.
|
 |
Amplatzer Amulet Left Atrial Appendage OccluderThe Amplatzer Amulet Left Atrial Appendage Occluder is the only FDA-approved LAA occluder that offers immediate freedom from OACs. With unique dual seal technology to ensure immediate and complete closure and the widest range of sizes of any LAA occluder, Amulet makes closure an option for more AFib patients. |
 |